Popular weight loss and diabetes drugs linked to increased risk of rare form of blindness
CNN || Shining BD
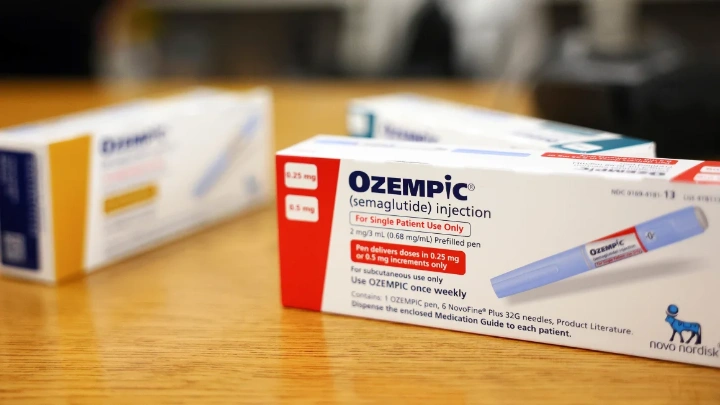
People who take Ozempic or Wegovy may have a higher risk of developing a rare form of blindness, a new study suggests. Still, doctors say it shouldn’t deter patients from using the medicines to treat diabetes or obesity.
Last summer, doctors at Mass Eye and Ear noticed an unusually high number of patients with non-arteritic anterior ischemic optic neuropathy, or NAION, a type of eye stroke that causes sudden, painless vision loss in one eye.
The condition is relatively rare — up to 10 out of 100,000 people in the general population may experience it — but the doctors noted three cases in one week, and each of those patients was taking semaglutide medications.
A look back at six years of medical records showed that people with diabetes were more than four times more likely to be diagnosed NAION if they were taking a prescription semaglutide, and those who were overweight or obese were more than seven times more likely to experience the condition if they were taking the medication. The risk was found to be greatest within the first year of receiving a prescription for semaglutide.
The study, published Wednesday in the medical journal JAMA Ophthalmology, cannot prove that semaglutide medications cause NAION. And the small number of patients — an average of about 100 cases were identified each year — from one specialized medical center may not apply to a broader population.
Novo Nordisk, the manufacturer of the only semaglutide medications in the US, emphasized that the data in the new study is not sufficient to establish a causal association between the use of semaglutide medications and NAION.
“Patient safety is a top priority for Novo Nordisk, and we take all reports about adverse events from the use of our medicines very seriously,” a company spokesperson wrote in an email to CNN.
Semaglutide prescriptions have soared in the US, which could raise the number of people at risk for a potential side effect. And NAION is the second-leading cause of optic nerve blindness after glaucoma.
But even with an increased risk, the condition remains relatively uncommon.
“The use of these drugs has exploded throughout industrialized countries and they have provided very significant benefits in many ways, but future discussions between a patient and their physician should include NAION as a potential risk,” lead researcher Dr. Joseph Rizzo, director of the neuro-ophthalmology at Mass Eye and Ear and a professor at Harvard Medical School, said in a news release. “Our findings should be viewed as being significant but tentative, as future studies are needed to examine these questions in a much larger and more diverse population.”
Experts agree that the potential risk of NAION should not deter the use of semaglutide medications to treat diabetes or obesity.
“In the ever-changing landscape of systemic therapies, being vigilant for potential new disease associations is a duty we all share on behalf of patients,” Susan Mollan, an ophthalmologist with the University Hospitals Birmingham in the UK wrote in a related commentary. But the large number of people who are taking semaglutide should raise confidence that the absolute risk of developing NAION as a result is rare.
The ways that semaglutides interact with the eyes are not entirely understood. And the exact cause of NAION is not known either. The condition causes damage to the optic nerve, but there is often no warning before vision loss.
Changes in blood sugar levels can affect the shape of an eye’s lens and may affect vision, said Dr. Disha Narang, an endocrinologist and director of obesity medicine at Endeavor Health in Chicago. She was not involved in the new study.
And the use of semaglutides, which prompt the body to create more insulin to reduce blood sugar, has been previously linked to temporary vision changes — new or worsening cases of diabetic retinopathy, or damage to blood vessels at the back of the eye — likely related to the rapid improvement of blood sugars.
The US Food and Drug Administration-approved labels for both Ozempic and Wegovy include vision changes among potential side effects, and Novo Nordisk is exploring the link between semaglutide use and diabetic retinopathy in a trial which they expect to complete 2027.
“Patient safety is paramount at the FDA and we continuously review available sources of data and new information on potential risks of drugs, including GLP-1 receptor agonists, and update labeling as needed to communicate new information on potential risks to healthcare providers and to patients as soon as possible,” Chanapa Tantibanchachai, a press officer with the FDA, told CNN over email.
While there is a “biologically plausible mechanism” for a potential interaction, “‘association is not causation’ and diabetes mellitus is a known risk factor for NAION,” Dr. Andrew Lee, clinical spokesperson for the American Academy of Ophthalmology and neuro-ophthalmologist at Houston Methodist Hospital, told CNN in an email. He was not involved in the new study.
For now, patients who are taking semaglutide or considering treatment should discuss the risks and benefits with their doctors, especially those who have other known optic nerve problems such as glaucoma or preexisting visual loss, experts say
“It is important to consult with ophthalmology if patients experience visual changes,” Narang said. “It is important to make sure patients are consulting with physicians who are also comfortable prescribing semaglutide and talking through what may be clinically relevant versus irrelevant, and discussing benefits versus risks of long-term therapy.”
Shining BD

![Why could a silent asthma epidemic be sweeping Africa? Sodiq Ajibade, 29, who suffers from asthma attacks, displays his medication in his home in Lagos, Nigeria [File: Temilade Adelaja/Reuters]](/Uploads/Images/News/2024/10/Image-14562-20241029062353.webp)

































